Strong Engagement for Pediatric Trials in Norway
A clinical trial on type 1 pre-diabetes was likely the topic that sparked the most engagement when NorPedMed recently brought together pediatric research staff from across the country.

NorPedMed, a national clinical research network for pediatric treatment, organizes such networking meetings annually. At this year’s full-day seminar, around 30 pediatricians, pediatric research nurses, and coordinators from all the university hospitals in Norway gathered in Oslo.
The agenda included topics such as international collaboration in pediatric trials, clinical trials on rare diagnoses, budgeting processes for studies, experiences with external inspections and audits, the pharmaceutical industry’s wishes and challenges, and, notably, the practical and ethical challenges of a clinical trial on pre-diabetes.
It was Henrik Irgens, senior consultant at the Children's Clinic at Haukeland University Hospital and head of the Medicines for Children Network, Norway, who presented on experimental treatment with immunotherapy for children diagnosed with pre-diabetes.
Around 400 children and adolescents are diagnosed with type 1 diabetes each year, but in the case of pre-diabetes, patients must be identified before they receive the diagnosis. So, how do we achieve that?
Camilla Tøndel spoke with great enthusiasm about her experience with external inspections and audits from both the DMP, FDA, and EMA, and offered her colleagues some advice on what should be well-organized—not only during clinical trials, but also for a long time afterward.
Olve Moldestad from Oslo University Hospital and Eli Bergli from Novartis spoke about the Partnership for Rare Diseases, which was established in 2024 as a public-private collaboration aimed at contributing both directly and indirectly to improved treatments for rare genetic diseases.
Senior consultant and pediatrician Johannes Rolin at the Vestfold Hospital Trust encouraged the university hospitals to include local hospitals in their industry-sponsored trials—not only to increase competence among healthcare professionals, but also, and perhaps most importantly, to make experimental treatments more accessible to a greater number of patients.
"Think of us—and include us! We have patients who would love to participate in studies. We don’t need to be a separate site, but we can carry out selected procedures and examinations under the leadership and responsibility of the main site," said Rolin. "We have both doctors and nurses who are eager to gain experience with clinical trials."
The network meeting was led by NorPedMed’s Chair and Coordinator, Professor Thomas Halvorsen and Senior Advisor Sigrun Margrethe Hjelle, who is also the pediatric coordinator in NorTrials.
Thanks to their encouragement and the engagement of the participants, one of the outcomes of the meeting is the establishment of a national network of pediatric research nurses, initiated by St. Olavs Hospital.
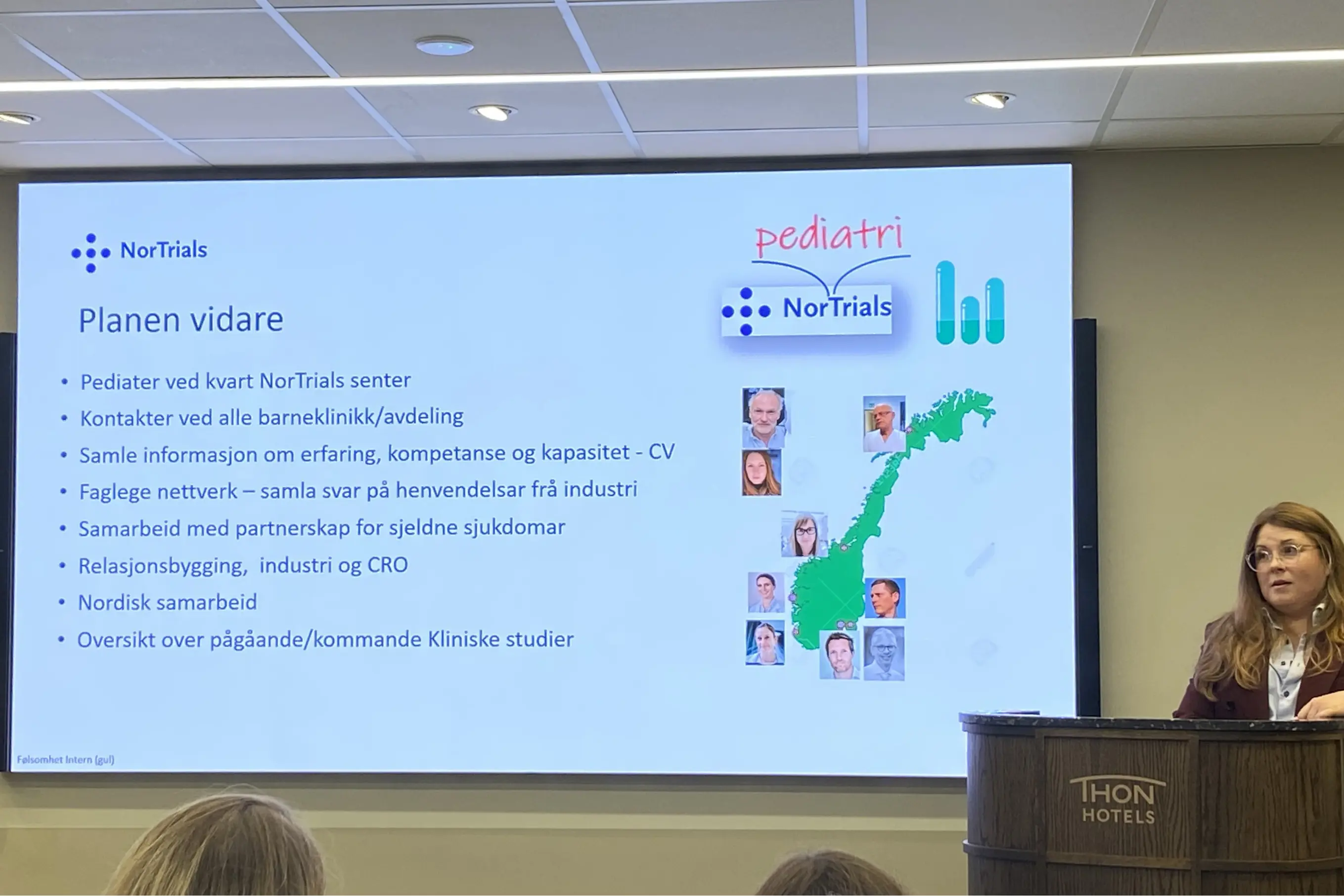
"Bringing together both doctors and research nurses from all the university hospitals is extremely valuable for everyone, and something we prioritize every year," says Hjelle. "We share experiences and challenges and offer each other both solid advice and inspiration for further development. There is strong willingness to take part in clinical trials for children and adolescents, and having research units with specialized personnel is absolutely essential to make this possible."
The article was translated from Norwegian to English using ChatGPT and edited by Signe Fretland.