NorTrials Medical Devices
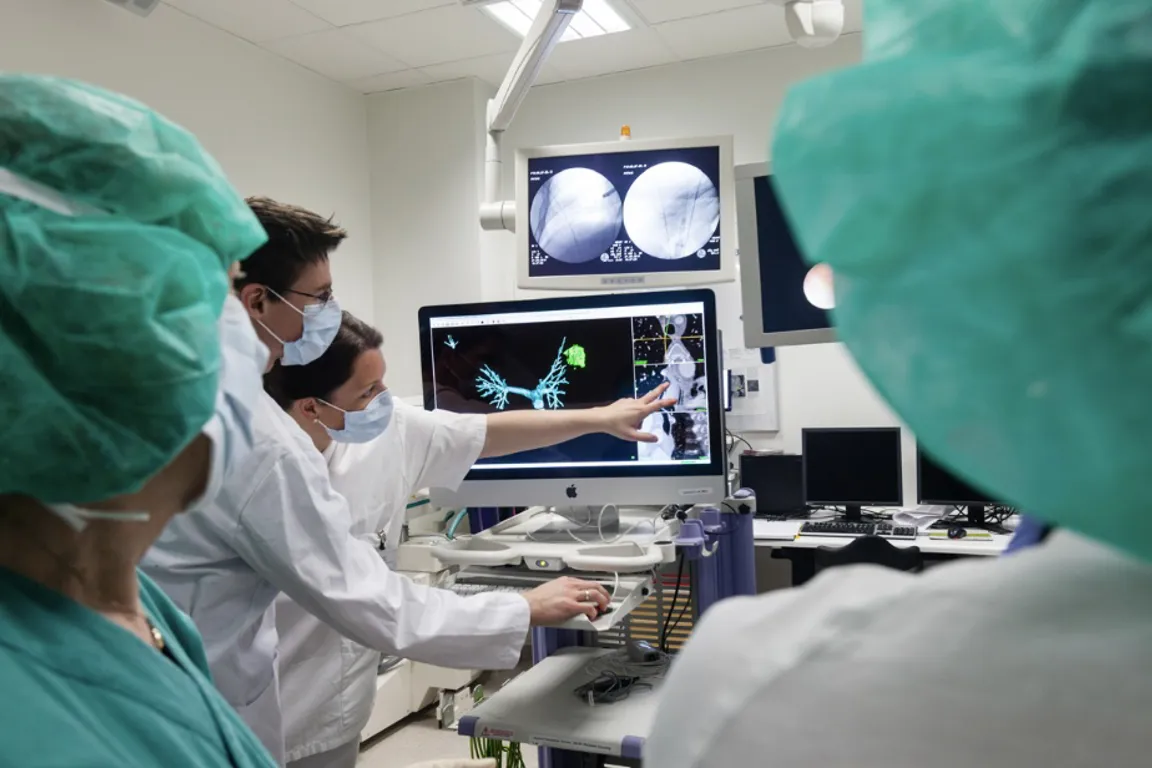
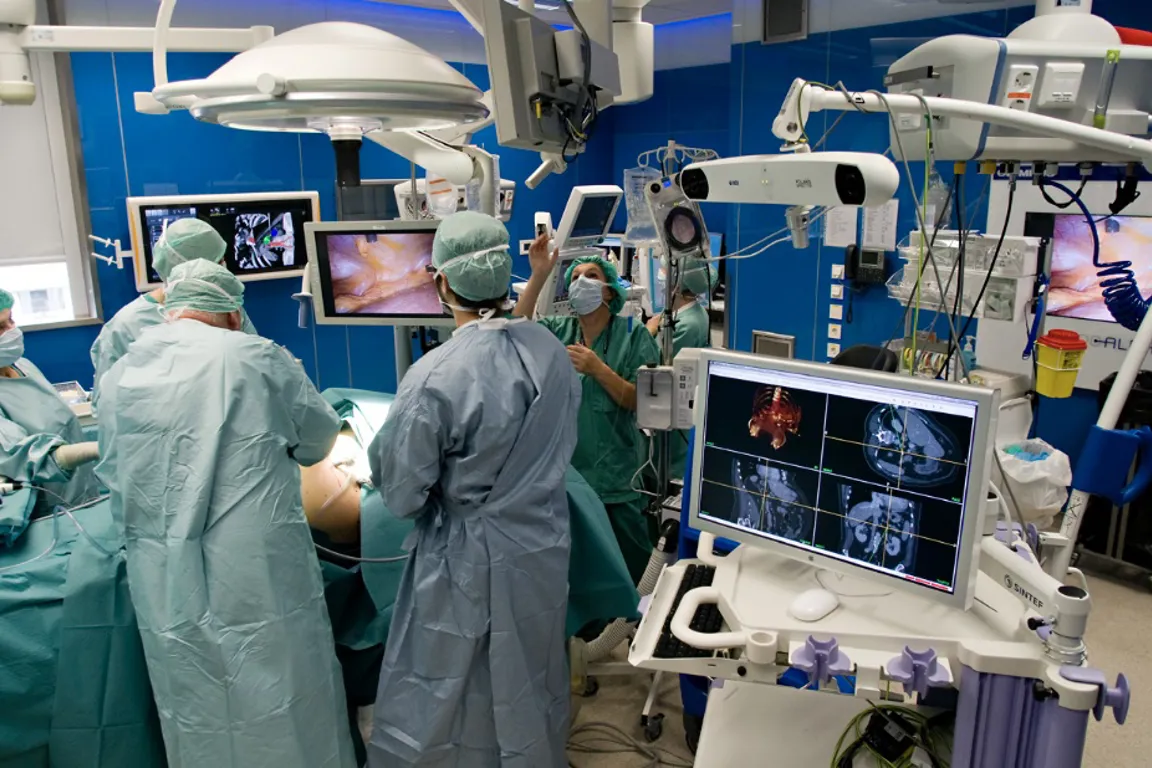
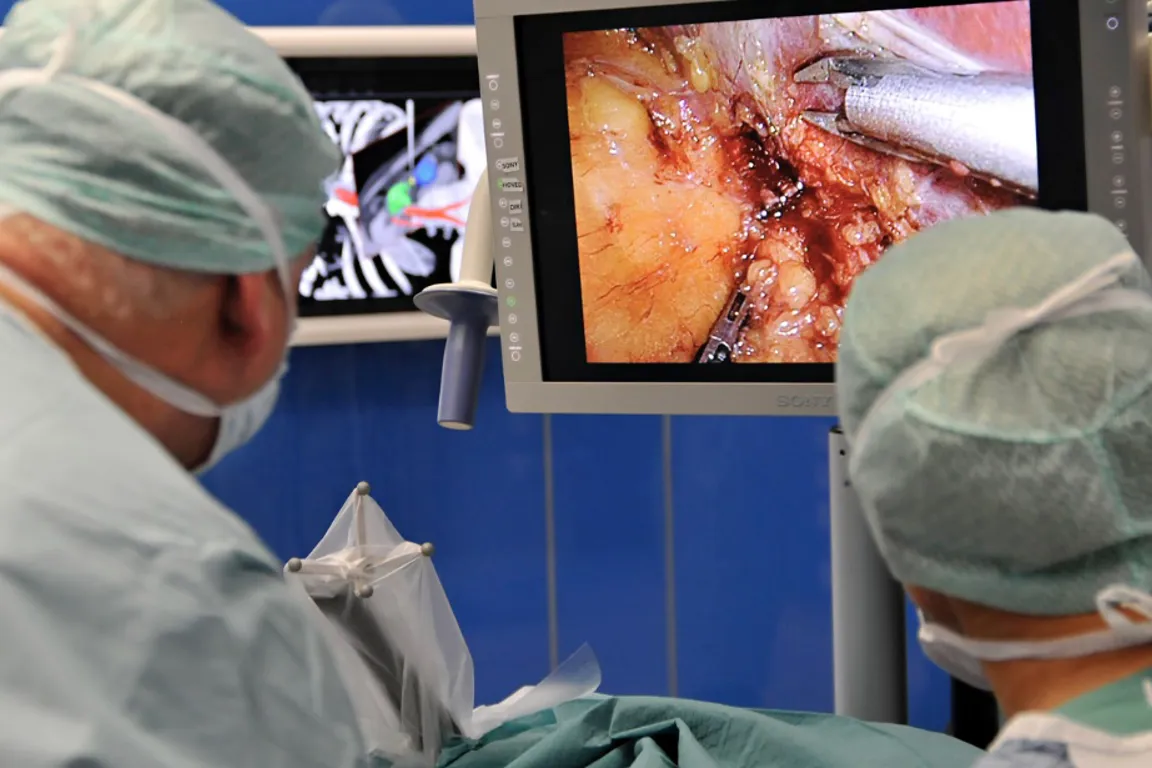
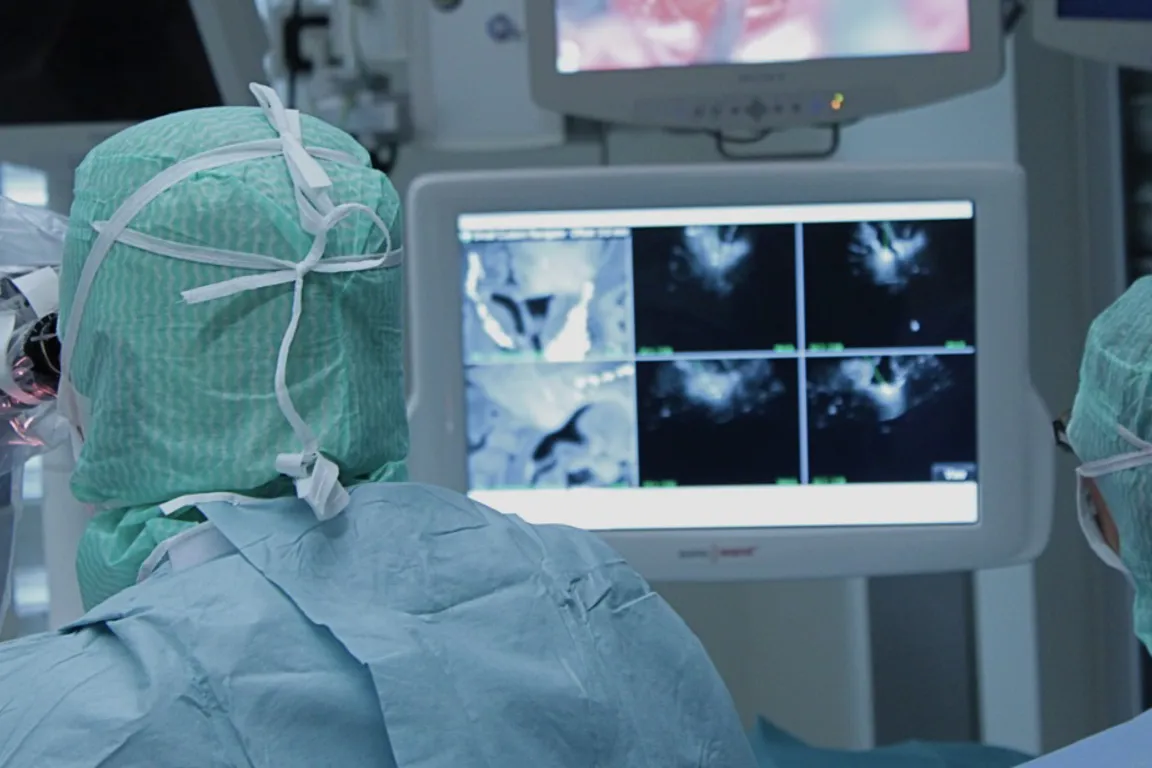
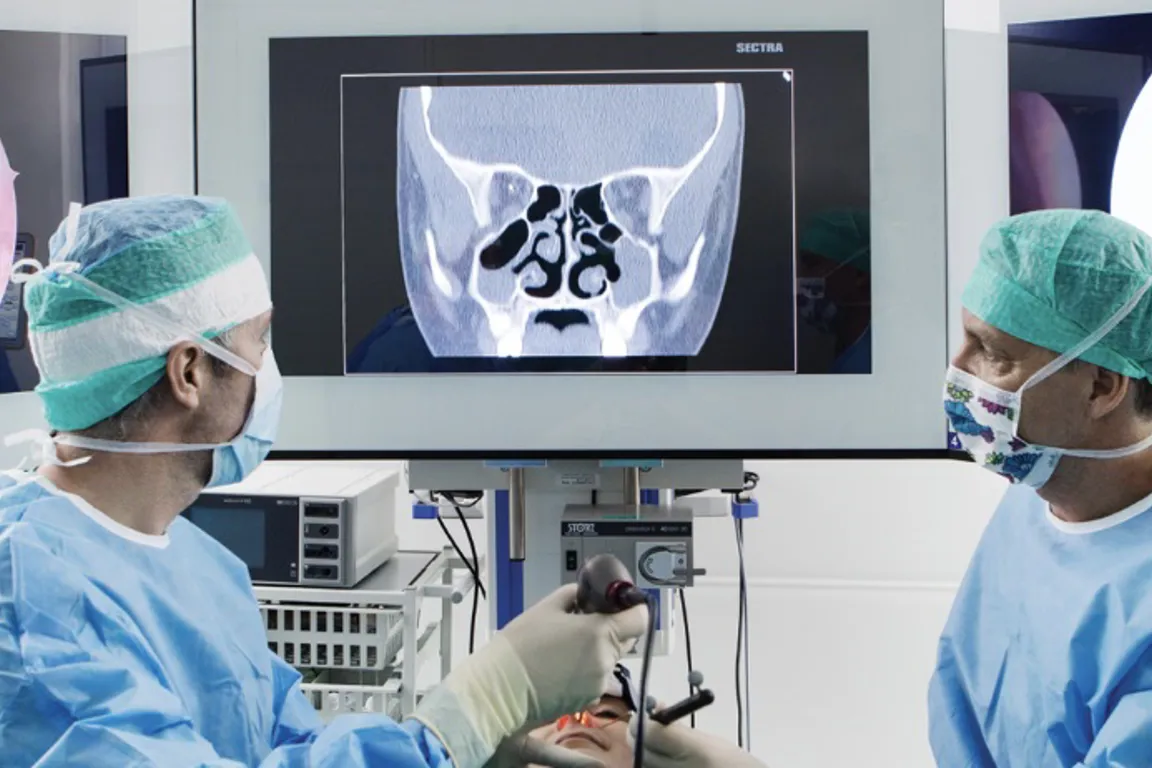
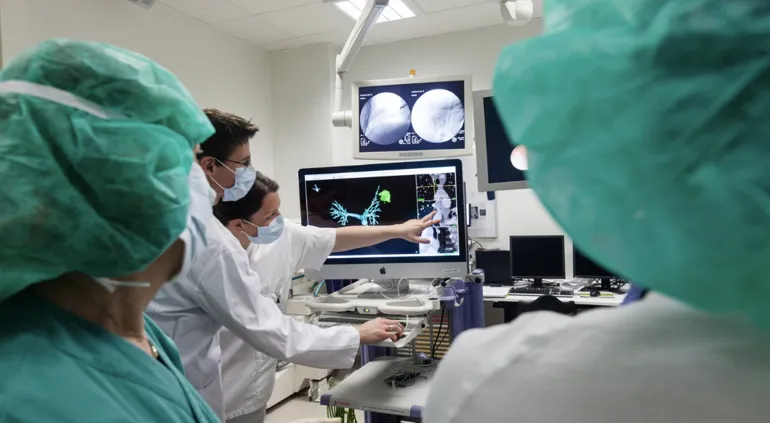
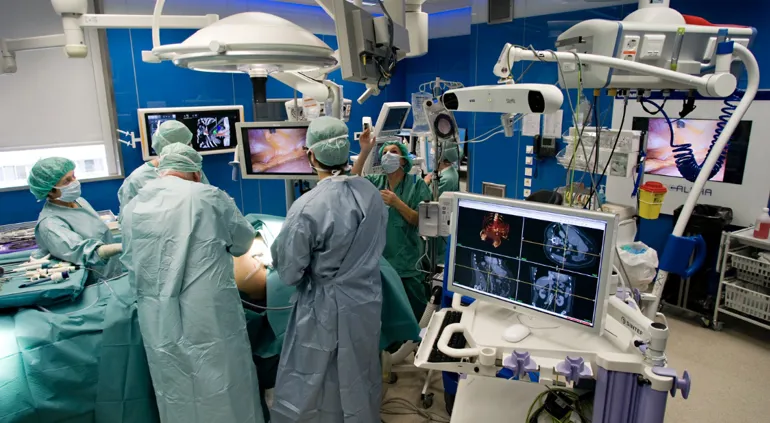
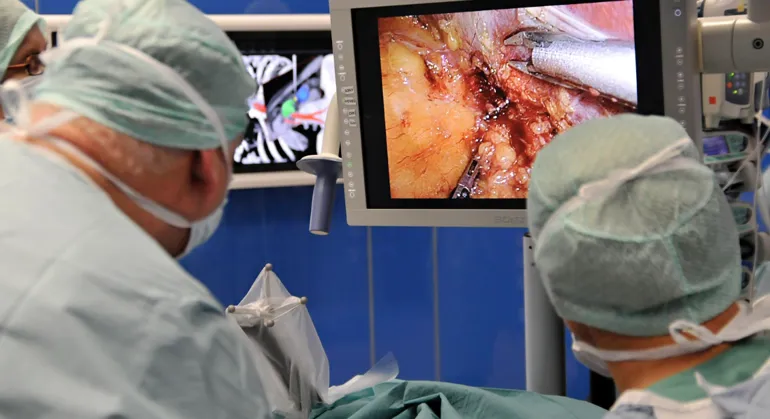
Use of medical devices is central to most diagnostics and treatment of patients in hospitals. Many hospitals engage in clinical trials comprising research, development and testing of medical devices (technology). The NorTrials centre for medical devices is located at St. Olavs hospital, Trondheim University Hospital.

St. Olavs hospital has extensive experience in research, development, innovation and testing of medical devices and technology in collaboration with industry.
The NorTrials centre for medical devices is organized in the research department and placed under
the research infrastructure "The Operation Room of the Future" (FOR), where we perform and support research, infrastructure, national roles, etc. in collaboration with the other health regions in Norway. One example is the NorMIT infrastructure in collaboration with Oslo University Hospital.
The NorTrials centre for medical devices will take this collaboration further and contribute to coordination and increased participation from more hospitals, small and large, across the country, in studies involving medical devices in collaboration with national and international industry.
Regulation (EU) No. 2017/745 on medical devices (MDR) entered into force on 26 May 2021. It means that the Norwegian Medicines Agency must assess applications for clinical trials/performance studies of medical devices in addition to ethical approval from the Regional Committees for Medical and Healthcare Research Ethics (REK).
In 2020, the first REK KULMU was established. The KULMU committees will handle pre-approval applications under the Medical Device Regulation, Clinical Trial Regulation and In-vitro Diagnostic Medical Device Regulation. REK KULMU works closely with the Norwegian Medicines Agency.
The centre collaborates with Health Catalyst to facilitate the testing of health technology
Centre Leader: Jan Gunnar Skogås
Medical Lead: Thomas Langø
Regulatory Advisor: Sara Edvardsen

Jan Gunnar Skogås
Jan