DMP can give you advice along the way
The road from research and clinical trials to a finished pharmaceutical product is winding and complicated. The Directorate for Medical Products (DMP) has an advisory and guidance service they encourage the industry to use.

A multidisciplinary team has been assembled to assist companies, start-ups, and academia with any questions they might have. The team is led by Ingvild Aaløkken, a special advisor on regulatory, clinical trials, and innovation at DMP, and includes advisors in the fields of veterinary medicine, medical devices, and regulatory matters. They can guide both companies and researchers onto the right path from research and innovation throughout the development and launch of new pharmaceuticals and medical devices.
How to contact DMP’s advisory and guidance service? Email ask-us@dmp.no or fill out the contact form at dmp.no.
The goal for this service is to provide the tools and knowledge needed from the idea phase to an approved product. In addition, this service provide companies and researchers access to expertise on various application processes, how to achieve the most robust data, how trials can be conducted with the best possible study design – and most importantly: how to get it right the first time and avoided having to repeat the process more than once.
- It helps save time and resources for patients, companies, and society, says senior advisor Kristina Berg Lorvik.
When an inquiry is received, DMP assembles an interdisciplinary team with relevant expertise for the specific elements in the injury and follows up through face-to-face meetings. DMP works hard to meet their internal processing timeline, which is set at approximately 6 weeks from submission of the inquiry to final response.
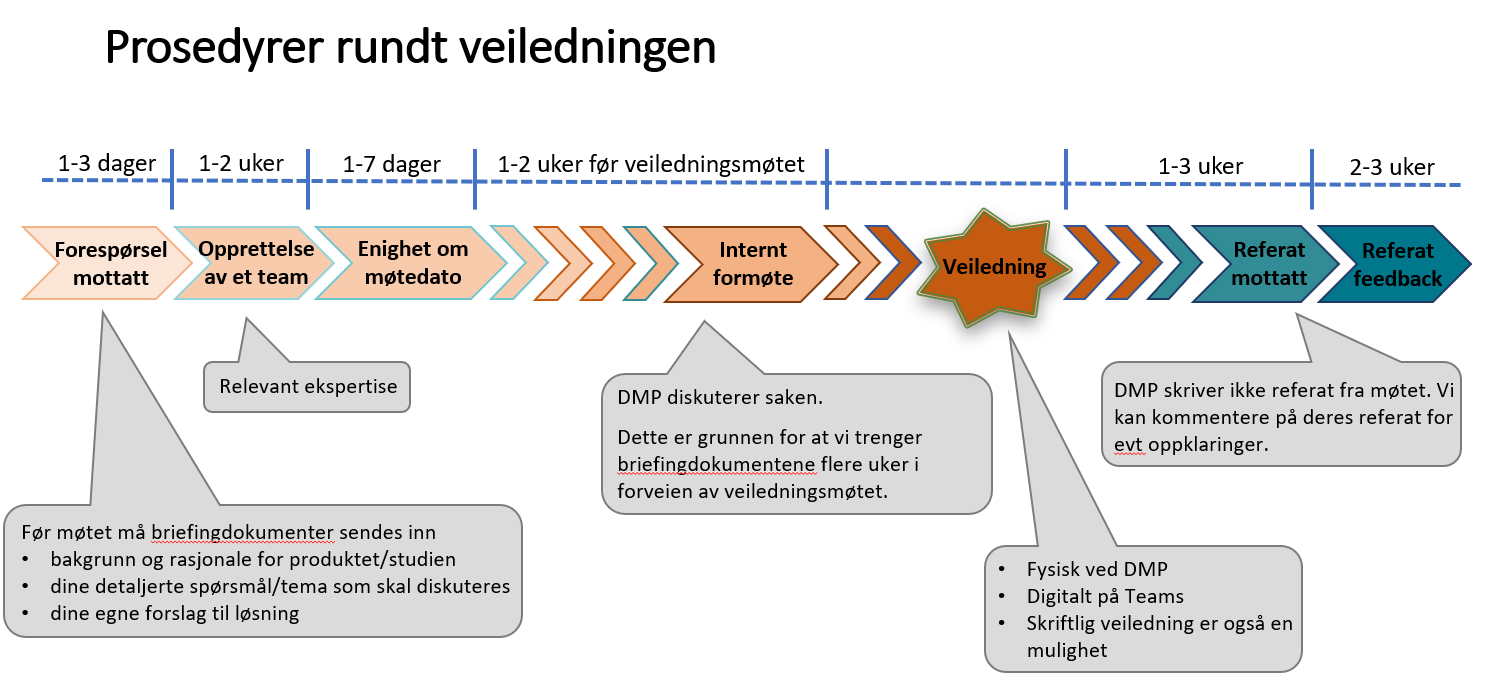
Occasionally there is a simple answer to a set rule or procedure, in which case they offer timely advice. Other times the inquiry is more complex and has several solutions in which DMP offers guidance and support in order to navigate through the submission process in the best-tailored way.
- Regarding pharmaceuticals, we can provide both advice and guidance, whereas for medical devices we only provide guidance, says Lorvik.
DMP offers these services to well-established global companies, start-ups, and academia.