Implementation of CTIS: - Challenging for Both Industry and Authorities
Following the introduction of the pan-European system; Clinical Trial Information System (CTIS), the process for submitting applications for clinical studies has become longer and more resource-intensive.

This is stated by, Anette Solli Karlsen, Senior Advisor at the Unit for Clinical Trials and Innovation at the Directorate of Medical Products (DMP).
- The time and resources, which is required for this new CTIS portal is much more demanding—it requires more recourses both in regards to employees and time than before the introduction of CTIS portal. The processing time has increased from 60 to about 106 days, there is less flexibility, strict deadlines, and in some cases, stricter documentation requirements than before. Particularly, the documentation related to national conditions has become more challenging for sponsors. CTIS portal, for example, is not suited for trials with a complex design, says Karlsen.
CTIS stands for Clinical Trials Information System and is a digital processing system introduced across Europe on January 31, 2022. It is a consequence of the Clinical Trials Regulation (CTR), which aims to harmonize the processing of clinical trial applications in Europe, increase the safety of trial participants, and ensure transparency around trial data.
- CTD = old regulation (regulated under the Clinical Trials Directive)
- CTR = new regulation (regulated under the Clinical Trials Regulation)
The intention is good, but the road to results is bumpy. The implementation has posed transitional challenges for researchers, clinicians, industry, and pharmaceutical authorities—including DMP.
- Until January 31, 2023, applications could be submitted under both regulations, but after this date, it is only possible to apply through the CTIS. The transition period has been particularly demanding for both the DMP and the industry. All ongoing trials submitted under the old regulation must be transferred to the new regulation with so-called transition applications, and companies are working very hard on this now. Estimates show that there are around 5,000 applications remaining to be transferred from CTD to CTR. We have completed about 20% so far, so we are curious to see how the next six months will unfold for us, says Karlsen.
Fortunately, Karlsen has confidence in that the situation will eventually improve.
- The fact that sponsors can now submit a single application valid across Europe, instead of having to apply in each country individually, will be a significant improvement. We at DMP also do not have to take the responsible role in each individual assessment, so this will be easier for us in some areas, says Karlsen.
For each application, the pharmaceutical authorities in one country is responsible for the assessment (RMS - Reporting Member State), while the other participating countries are co-responsible (MSC - Member State Concerned). In Norway, the DMP and the Committees for Clinical Trials of Medicinal Products and Medical Devices (REK KULMU) will collaborate on the evaluation of the trials.
Applications are divided into two parts. Part 1, for which the DMP has coordination responsibility, protocol, risk/benefit, manufacturing and import, labeling, etc., are assessed. REK KULMU and DMP conduct a joint assessment of part 1 on behalf of Norway. In part, 2 for which REK KULMU is responsible, consent forms, recruitment, data protection, the suitability of the trial site, use, and storage of biological material, among other things.
Part 1 can be submitted independently of Part 2, however if there is an ongoing application in CTIS portal, Part 2 cannot be submitted until all previous applications are fully processed. It is not possibly to change or add documents after the application is submitted. Due to this, most sponsors submit both part 1 and 2 simultaneously.
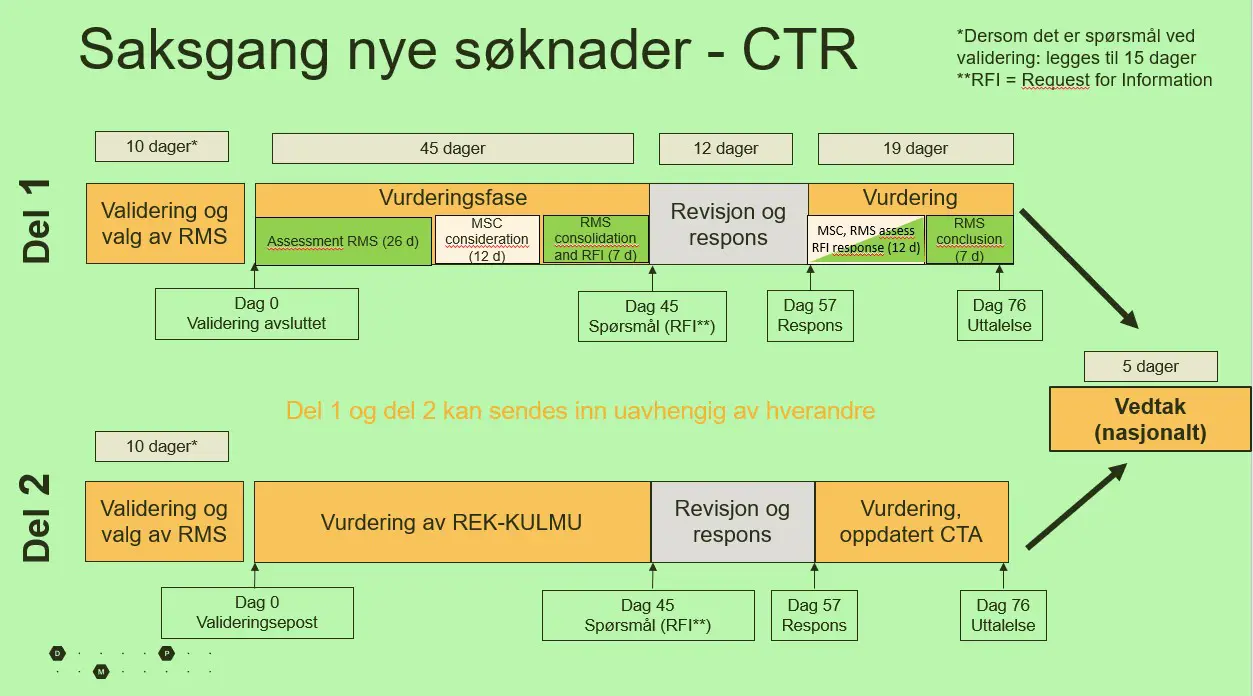
Figure 1: All documentation will be assessed within designated timeframes.
- All public holidays of the responsible evaluating country (RMS) must be taken into account before submission, so with different holidays and days off in various countries, the deadlines can become quickly drawn out. If the sponsor response before their 12 – day deadline, we must response within out allotted 19 days from the day they have replied. The entire process demands a lof from both us as authorities and from the sponsor. However, we believe that this will eventually settle and become easier for both parties involved, says Karlsen.
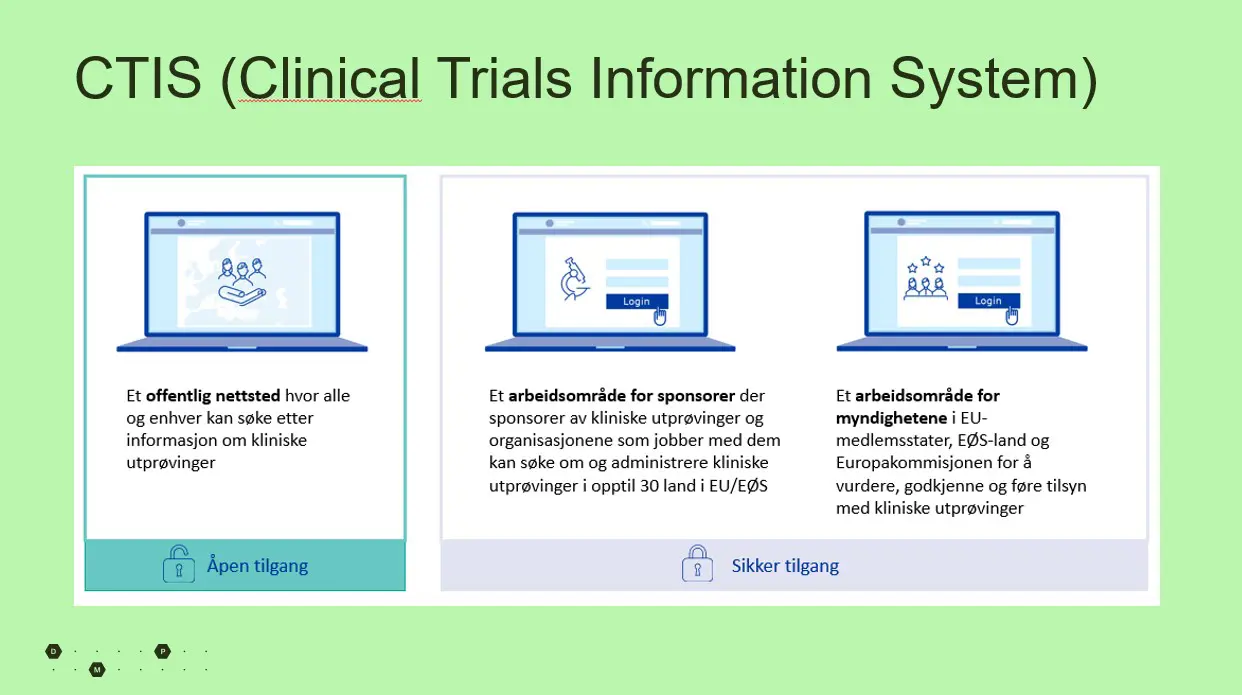
Figure 2: CTIS is a public website where anyone can search for information about clinical trials approved by the pharmaceutical authorities. In addition, there is a secure area of CTIS where sponsors and the authorities have accesses. The latter part is discussed in this article above.